Chemical equivalent of arene oxides and oxepines: substitution patterns in nitrogen Heterocycles. A case study in the U.S.
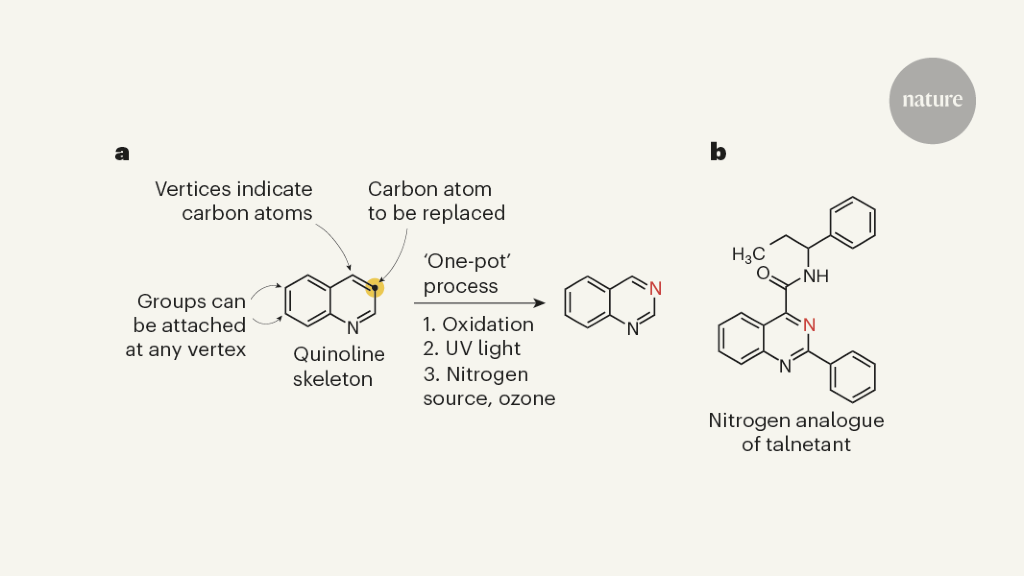
The molecule of the active ingredients in pharmaceuticals and agrochemicals is largely composed of carbon atoms, but they also contain other atoms such as nitrogen, oxygen and sulfur. It’s a good idea for chemists to change the skeletons with another element so they can create analogues of biologically active compounds. However, this conceptually straightforward and potentially versatile transformation is missing from much of the extensive repertoire of reactions used for organic synthesis. An example of a single-atom substitution is provided in a paper by Woo et al.1, that talks about replacing a carbon atom with a nitrogen atom in quinolines.
Schönherr, H. & Cernak, T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. There is a Chem. Int. The Ed. 52 was published in the year 2013).
The necessary nitrogen atom is a high impact design element. J. Med. 60, 3554–5579 was published in 2017).
The structural diversity, substitution patterns, and frequencies of nitrogen Heterocycles in the U.S. are analyzed. The J. Med. is a specialty journal. Chem. 57, 10257–10274 (2014).
From the beginning of the medicine to the end is the therapeutic chemistry perspective. Bioorg. There is a doctor. A chemical substance. Lett. 26, 3381–3394 (2016).
A book on Successful Drug Discovery was published in 2015.
Kelley, B. T., Walters, J. C. & Wengryniuk, S. E. Access to diverse oxygen heterocycles via oxidative rearrangement of benzylic tertiary alcohols. There is an organization called Org. Lett. 18, 1896–1899 (2016).
Siddiqi, Z., Wertjes, W. C. & Sarlah, D. Chemical equivalent of arene monooxygenases: dearomative synthesis of arene oxides and oxepines. J. Am. Chem. Soc. 142, 10125–10131 (2020).
Skeletal Editing using Direct Nitrogen Deletion. Nature’s Reports for the Period Between January 2011 and March 2011. Org. Lett. 23, 6126 & 6130
Kennedy, Dherange, B. D., Berger, K.J., and M. D. Skeletal editing using direct nitrogen deletion. The following are the Nature’s reports for the period from January to March 2011.
The addition of Boron to alkyl ether bonds via zinc and nickel tandem catalysis was reported in Lyu, H. Science 372, 175–2 will be published in 2021.
Morofuji, T., Inagawa, K. & Kano, N. Sequential ring-opening and ring-closing reactions for converting para-substituted pyridines into meta-substituted anilines. Org. In Lett. 23, 6 126–6130, there are depictions of the year 2020.
Reisenbauer, J. C., Green, O., Franchino, A., Finkelstein, P. & Morandi, B. Late-stage diversification of indole skeletons through nitrogen atom insertion. Science 377, 1104–1109 has been published.
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
Photolysis and bond activation of pyridines in diethylamine. A story of a cut and sew-it-and-lose transformation
Sundberg, R. J., Suter, S. R. & Brenner, M. Photolysis of 0-substituted aryl azides in diethylamine. The formation of the two-diethylmino-1H-azepine intermediates. J. Am. Chem. In 1972 the Soc. 94, and 525-528 were published.
Chen, P., Billett, B. A., Tsukamoto, T. & Dong, G. ‘Cut and sew’ transformations via transition-metal-catalyzed carbon–carbon bond activation. A version of the story was published in the ACS Catal 7.
Boyle, B. T., Levy, J. N., de Lescure, L., Paton, R. S. & McNally, A. Halogenation of the 3-position of pyridines through Zincke imine intermediates. Science 378, 773–779 (2022).
A, Wilson, G.G. and Murray, N. E. discussed the highlights of the DNA cutter. Nucleic Acids Res. 42, 3–19 (2014).
Willand-Charnley, R., Fisher, T. J., Johnson, B. M. & Dussault, P. H. Pyridine Is an organocatalyst for the reductive ozonolysis of alkenes. There is an authorized organization. Lett. 14, 2225–2235.
C8selective acylation of quinolineN-oxides with -oxocarboxylic acids is done through regioselective C–H bond activation. There is an organization. Lett. 18, 3722–3825.
Shieh, P., Hill, M. R., Zhang, W., Kristufek, S. L. & Johnson, J. A. Clip chemistry: diverse (bio)(macro)molecular and material function through breaking covalent bonds. Chem. Rev. 121, 7059–7121 (2021).
Cochran, B. M. A green ozonolysis–Pinnick tandem transformation is a commercial process to prepare AMG 232. J. Org. Chem. 84, 4763–4779 (2019).
Ragan, J. A. et al. Safe execution of a large-scale ozonolysis involves preparation of the 2-hydroxyindan-2-carboxaldehyde adduct. There is a Process Res. There is a report in the Development 7, 155–160
Source: Carbon-to-nitrogen single-atom transmutation of azaarenes
Ozonolysis of dimethoxyethene and vinyl acetate in the neurokinin 3 Receptor transmembrane domain
Malherbe, P. There are binding pockets in the human tachykinin neurokinin 3 Receptor transmembrane domain, but not identical binding pockets. The monograph on pharmaceutica was published in 2008
Dexter, D. L. et al. The activity of a novel 4-quincarboxylic acid. There are experimental tumors that contain [6-fluoro 2-22-yl]-7-benzyl-8-yl)-2-biphenyl-4-yl]-methyl-4-quinoline-carboxylic acid salt. In 1985 the Cancer Res 45 was published.
Wojciechowski, B. J., Chiang, C. Y. & Kuczkowski, R. L. Ozonolysis of 1,1-dimethoxyethene, 1,2-dimethoxyethene and vinyl acetate. J.Org is an organization. Chem. 55, 1120–1122 (1990).
Gollnick, Koegler and other researchers studied the thermal transformation of oxazole endoperoxides. The Lett. 29, 1007–1020, was published by the Tetrahedron Lett.
Kohlmeyer, C., Schäfer, A., Huy, P. H. & Hilt, G. Formamide-catalyzed nucleophilic substitutions: mechanistic insight and rationalization of catalytic activity. ACS Catal. 10, 11567–11577 (2020).